Precipitation of Aluminum Hydroxide From Aqueous Solution
Cuneyt Tas Peter J. Write the net ionic equation for the precipitation of barium carbonate from aqueous solution.

Types Of Chemical Reactions Chemistry Education Chemical Reactions Chemistry Class
Use the solubility rules provided in the OWL Preparation Page to.
. The role of oligomeric aluminate species in the precipitation of aluminum Al phases such as gibbsite α-AlOH 3 from aqueous hydroxide solutions remains unclear and difficult to probe directly despite its importance for developing accurate predictions of Al solubility in highly alkaline systems. See the answer See the answer done loading. The principal aluminum species detected in acid solutions by computer analysis of potentiometric data were AlOHand Al8OH2j.
The process involves first a nucleation stage to form primary seeds by adding an acidic aluminium sulphate solution to a Bayer plant liquor which contains 210 gL. Ag aq OH- aq. The concentrations of heavy metals in the synthetic wastewater range from 1 to 14 mgL for lead 5 to 90 mgL for zinc 3 to 90 mgL for copper and 5 to 45 mgL for iron.
Write the net ionic equation for the precipitation of aluminum hydroxide from aqueous solution. Experts are tested by Chegg as specialists in their subject area. Who are the experts.
SUMMARY Aluminum oxide has been prepared by thermal dissociation of alu- minum hydroxide obtained by precipitation with formalin from sodium. We review their content and use your feedback to keep the quality high. The hydroxide precipitation and coagulation-flocculation methods were used to treat wastewater containing lead zinc copper and iron.
Solution tank aluminum hydroxide precipitation particles Prior art date 1951-09-22 Legal status The legal status is an assumption and is not a legal conclusion. The region of precipitation of aluminum hydroxide by mixing the aqueous solutions of aluminum chloride 003200 mM and sodium hydroxide pH 35115 was investigated tyndallometrically at 20C 24 hr after mixing at varied ionic strengths. The precipitation of aluminium hydroxide is promoted by bubbling carbon dioxide gas into the supersaturated sodium aluminate solution ie the carbonation process Misra 1986 Zhao et al 2004 meanwhile the caustic in the sodium aluminate solution is converted to sodium carbonate which is recycled to extract alumina from the bauxite in the sintering process.
We review their content and use your feedback to keep the quality high. Soc 85 6 142129 2002 journal Synthesis of Gallium Oxide Hydroxide Crystals in Aqueous Solutions with or without Urea and Their Calcination Behavior A. Hydrotalcite-like composite synthesized by co-precipitation method was used as an adsorbent to remove the sulfate ions in aqueous solution.
On this day in 1953 Ralph W. You must be signed in to discuss. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed Expired - Lifetime Application number US247861A Inventor Ralph W Brown.
The process involves first a nucleation stage to form primary seeds by adding an acidic aluminium sulphate solution to a Bayer plant liquor. Okay Im assuming were looking for a chemical equation here because theres no actual question. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds Submit Answer Retry Entire Group 1 more group attempt remaining Write the net ionic equation for the precipitation of aluminum hydroxide from aqueous.
Majewski and Fritz Aldinger Max-Planck-Institut fuer Metallforschung Pulvermetallurgisches Laboratorium Stuttgart D-70569 Germany Gallium oxide hydroxide GaOOH䡠xH2O single. The coprecipitations were monitored by potentiometric pH titration and the. Aqueous solutions of aluminum chloride and sodium hydroxide are mixed forming the precipitate aluminum hydroxide.
Brown was granted the patent for PRECIPITATION OF ALUMINUM HYDROXIDE. By adding a 40 wt NaOH solution aluminum was removed from the wastewater as a hydroxide by precipitation particles of 10-90 lm are expected Andersson and Hansson 2001. Previous question Next question.
US4049773A US05675216 US67521676A US4049773A US 4049773 A US4049773 A US 4049773A US 67521676 A US67521676 A US 67521676A US 4049773 A US4049773 A US 4049773A Authority US United States Prior art keywords precipitation stage aluminum hydroxide suspension mother liquor Prior art date 1975-04-16 Legal status The legal status is an. Write the net ionic equation for the precipitation of calcium hydroxide from aqueous solution. Abstract The preparation of gelatinous aluminium hydroxide from aqueous solutions of the formate citrate or tartrate of aluminium on addition of aqueous sodium hydroxide has been investigated unde.
XRD FT-IR SEM and EDS elemental analysis were used to clarify the structure and composition of the hydrotalcite-. Magnesium aluminium hydroxides were coprecipitated from different mixed metal cation solutions at total CM 01 M and MgAl2ratios from 1 to 6 with sodium hydroxide solution at ambient temperature with different pre-ageing conditions for the aluminium hydroxide pre-precipitate. Write the net ionic equation for the precipitation of aluminum phosphate from aqueous solution.
100 7 ratings Transcribed image text. Request PDF Rapid and efficient chromium VI removal from aqueous solutions using nickel hydroxide nanoplates nNiHs Chromium VI water contamination still represents a. Experts are tested by Chegg as specialists in their subject area.
Write the net ionic equation for the precipitation of aluminum phosphate from aqueous solution. And amorphous AlOHgc A colloidal aluminum hydroxide hydrosol formed in acid solutions up to hydroxidealuminum ratios of 30 and a settleable precipitate formed between 30 and 40. More 40 wt.
This is the best answer based on feedback and ratings. The principal method used heretofore in the production of aluminum hydroxide has been to dissolve alumina from bauxite by means of an aqueous caustic soda solution and then precipitate aluminum hydroxide by auto-precipitation. This problem has been solved.
Write the net ionic equation for the precipitation of iron III sulfide from aqueous solution. Write the net ionic equation for the precipitation of nickel II hydroxide from aqueous solution. View the full answer.
The precipitation of fine aluminium hydroxide Al OH 3 powders average particle size 2 μm from Bayer plant liquor was investigated which is important for the production of specialty alumina Al 2 O 3 products. The precipitation of fine aluminium hydroxide Al OH 3 powders average particle size 2 μm from Bayer plant liquor was investigated which is important for the production of specialty alumina Al 2 O 3 products. Write the net ionic equation for the precipitation of calcium sulfide from aqueous solution.

Solved Write The Net Ionic Equation For The Precipitation Of Chegg Com
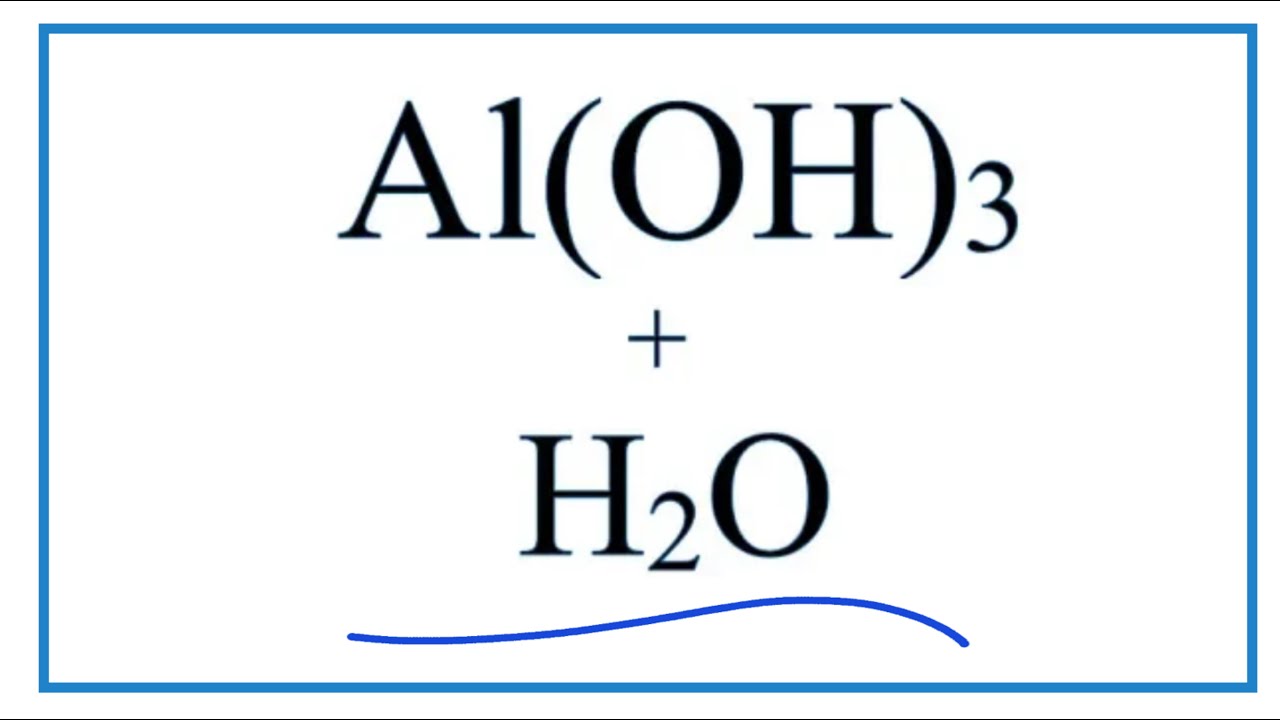
Equation For Al Oh 3 H2o Aluminum Hydroxide Water Youtube

Solved Aqueous Solutions Of Aluminum Chloride And Sodium Hydroxide Are Mixed Forming The Precipitate Aluminum Hydroxide
No comments for "Precipitation of Aluminum Hydroxide From Aqueous Solution"
Post a Comment